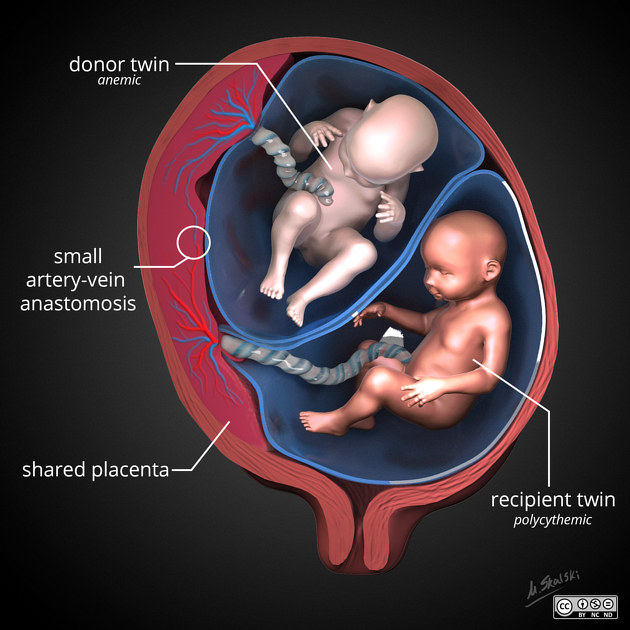
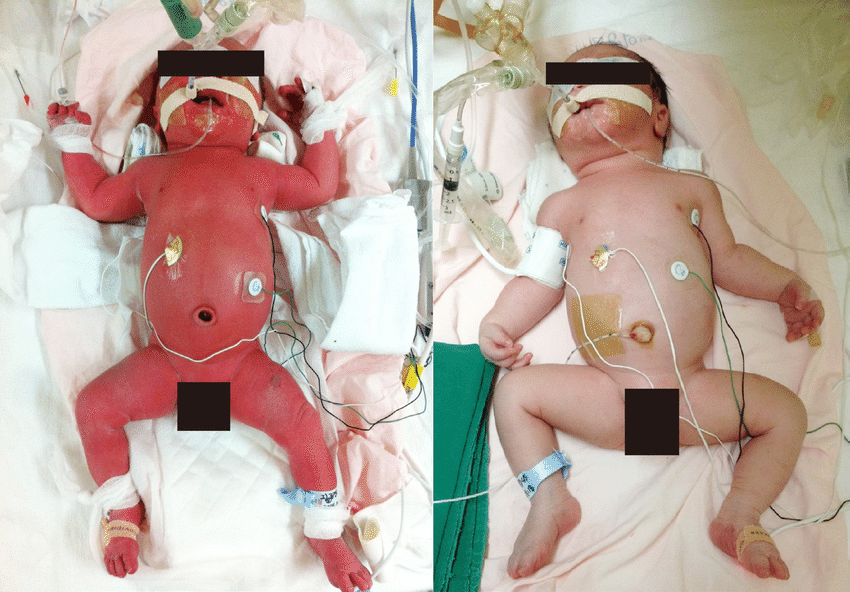
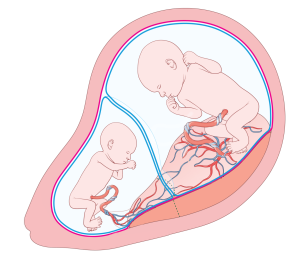
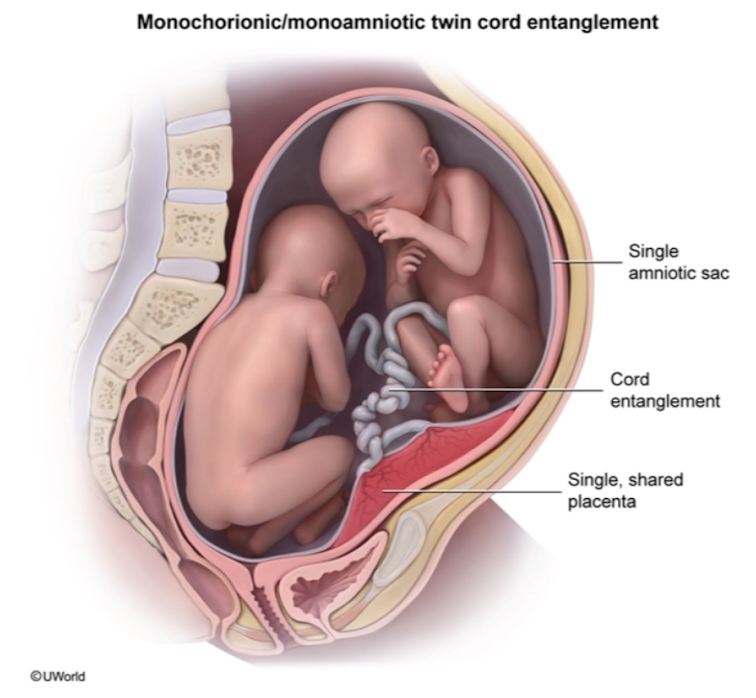
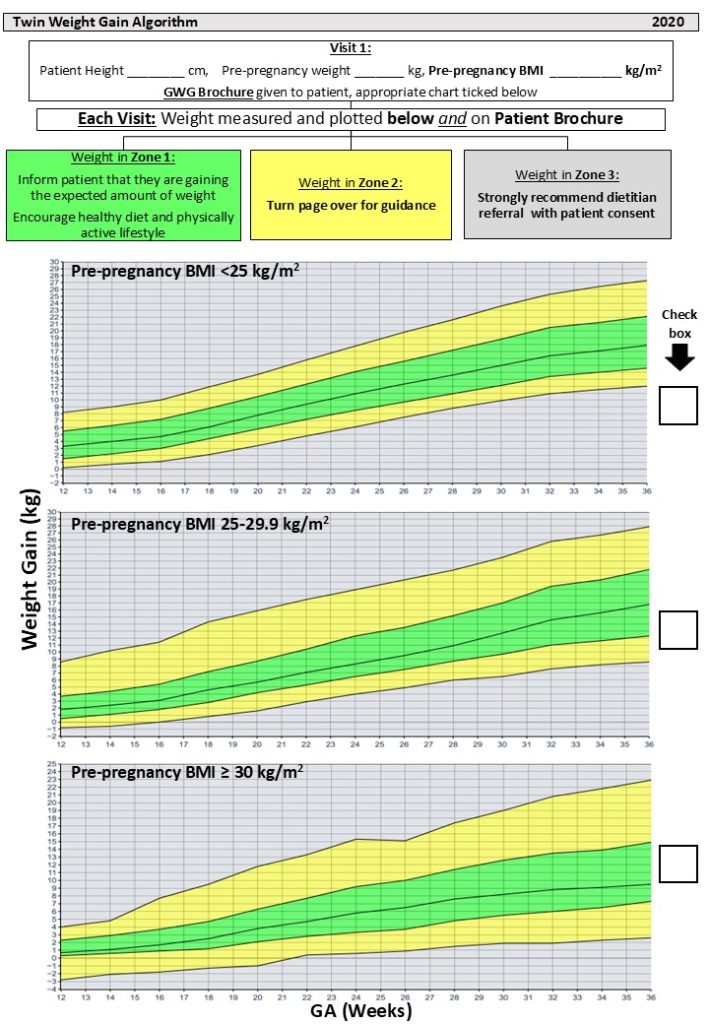
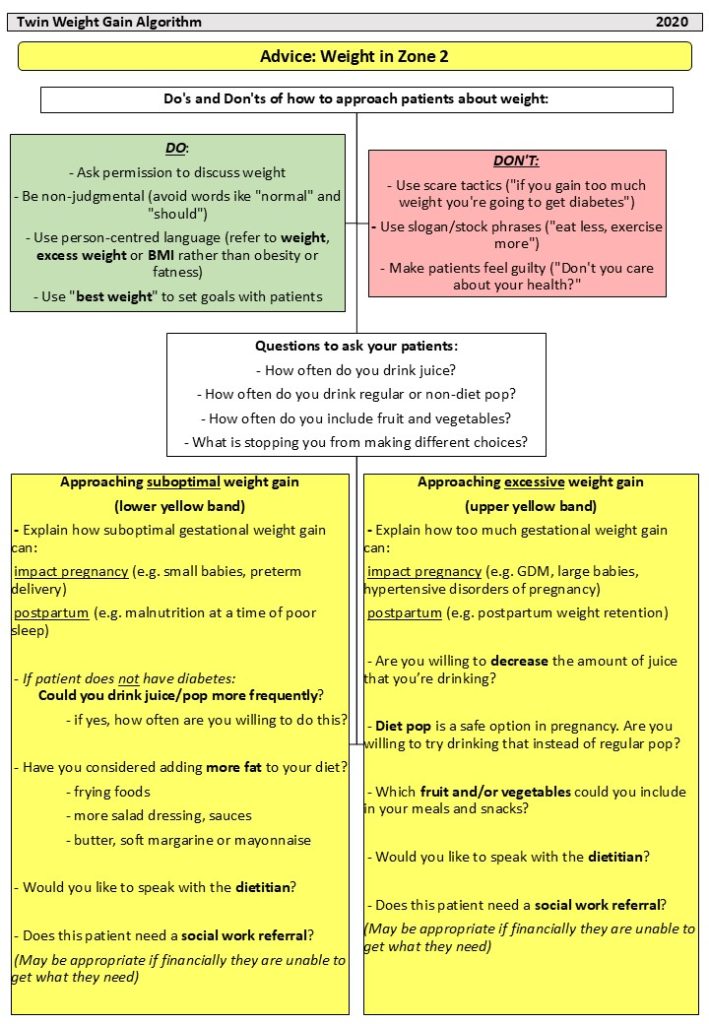
Preeclampsia is a condition that is characterized by hypertension (high blood pressure) with proteinuria (protein in the urine) and/or injury to other organs, such as the liver or kidneys. In its most severe forms, it can also cause seizures (known as eclampsia). It is thought to be caused by a failure of the maternal blood vessels that supply the placenta (the spiral arteries) to adjust properly to pregnancy, resulting in insufficient blood supply to the placenta.
Epidemiology
Up to 10% of mothers with twins will be diagnosed with a hypertensive disorder of pregnancy, including either gestational hypertension or preeclampsia. Twin mothers are more than 2 to 4 times more likely to develop this disorder compared with singleton pregnancies. A study from our group found that 14.4% of twin mothers in Ontario developed a hypertensive disorder of pregnancy, compared with only 1.6% of mothers with a singleton pregnancy.
Risks of preeclampsia
Preeclampsia can be dangerous for both the mother and her babies. Babies can become growth-restricted, be born preterm, or have low amniotic fluid (oligohydramnios). Mothers are at risk of life-threatening complications, including liver and kidney injury, placental abruption, and development of seizures (eclampsia), which is an obstetrical emergency.
You are at higher risk of developing preeclampsia if you were diagnosed with preeclampsia during a previous pregnancy, if you are older, or if you have preexisting conditions such as chronic hypertension, kidney disease, or high body mass index. See the section below on prevention for a better understanding of how we can reduce the risk of recurrence of preeclampsia.
Diagnosis
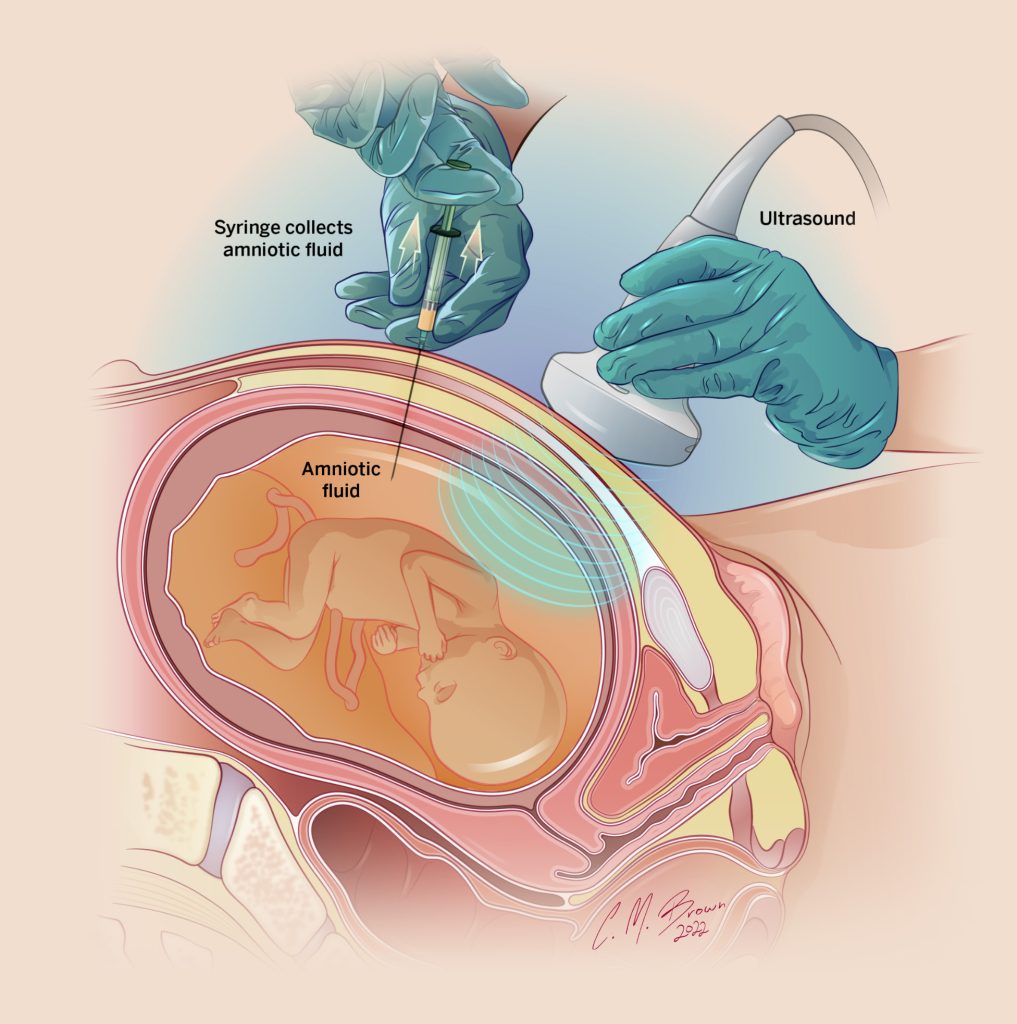
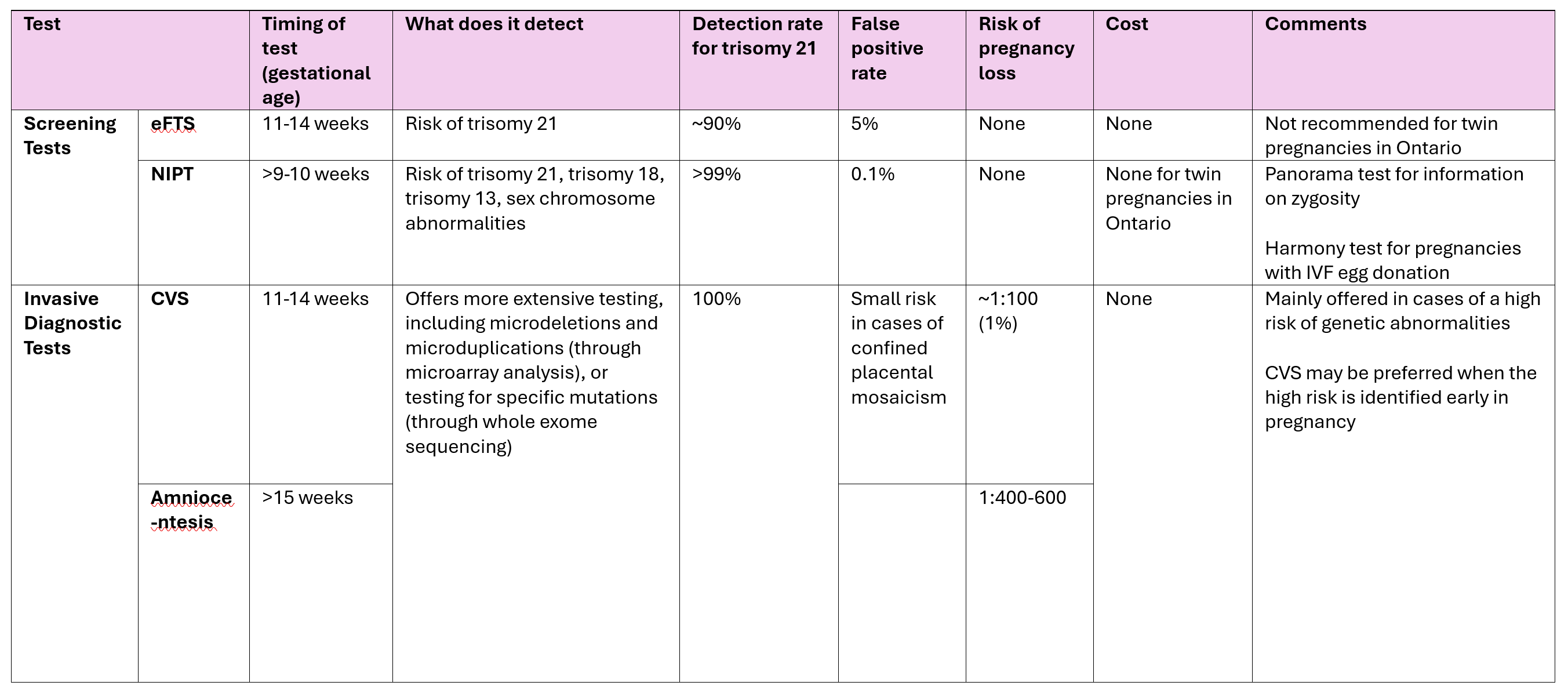
Most mothers will be asymptomatic when they are diagnosed with preeclampsia and are found by chance to have high blood pressure and protein in the urine (proteinuria). In these cases, we would usually send blood work (such as blood count and liver and kidney function tests) to determine the severity of preeclampsia. In addition, Sunnybrook is one of the few centers in Ontario that has a special test (sFlt-1/PlGF ratio) that can confirm (or rule out) the diagnosis of preeclampsia in unclear cases and predict the risk of it becoming more severe.
Symptoms that should make you immediately seek medical attention include severe headache, blurry vision, abdominal pain, confusion, or shortness of breath.
Management of preeclampsia
The only cure for preeclampsia is delivery of the babies and the placenta(s). Management of preeclampsia depends on the severity of the disease, with the overall goal being to defer delivery as much as possible to minimize the risks of prematurity to the babies, while balancing the risks of the condition to the mother.
In cases of mild preeclampsia, delivery is commonly deferred until 37 weeks. Until then, we advise mothers to monitor their blood pressure at home and come to the hospital if the blood pressure increases above 150-160 mmHg systolic or 95-100 mmHg diastolic, or if they experience symptoms such as headache, blurry vision, or epigastric pain.
Blood pressure medications are often prescribed to control blood pressure levels (most commonly a drug called labetalol). These medications are safe in pregnancy! We also monitor the mother’s blood work periodically to check liver and kidney function to make sure that the preeclampsia hasn’t evolved to a more severe form.
In cases of severe preeclampsia, delivery may be indicated earlier, especially if the blood work becomes abnormal. HELLP syndrome (which stands for Hemolysis, Elevated Liver enzymes, and Low Platelets) is the most severe form of preeclampsia (although there is some academic debate as to whether they are two different conditions). Symptoms most commonly start between 28 to 37 weeks of gestation, but can also start later in pregnancy, and 30% of cases develop post-partum. Rest assured that this is an uncommon complication.
Can preeclampsia be prevented?
Research has shown that starting to take low-dose aspirin before 16 weeks of gestation (162 mg daily at bedtime) can substantially reduce the risk of severe preeclampsia. We will usually recommend aspirin if you have additional risk factors for preeclampsia, such as age over 35 years, elevated BMI, history of hypertension, diabetes, or a history of preeclampsia in a prior pregnancy. Ask your obstetrician before starting any medication.
I have preeclampsia, what should I do?
Regularly check in with your obstetrician for monitoring and know the signs which mean you should go seek medical attention immediately: severe or persistent headache, visual changes, new shortness of breath, right upper quadrant or epigastric pain. Additionally, any decrease in fetal movement, vaginal bleeding, abdominal pain, rupture of membranes, or regular uterine contractions should also be a sign to go to your nearest medical centre.
How is preeclampsia in twin pregnancies different than singleton ones?
- Preeclampsia is more common in twin pregnancies.
- Mothers with preeclampsia may be at higher risk of future cardiovascular disease; however, our group has demonstrated that this less likely to be the case for mothers who had preeclampsia in a twin pregnancy compared with those who had preeclampsia in a singleton pregnancy.